T lymphocytes are cells of the adaptive immune system that play a central role in the immune defense against pathogens, in generating an immunological memory, and in maintaining tissue homeostasis and function. Importantly, T cells are among immune cell populations most detrimentally affected by aging. Our studies of the metabolic regulation of T lymphocytes would lead to the development of novel therapeutic approaches to target dysregulated immune responses in aging and disease.
Discover metabolic regulators of T cell activation
T lymphocytes survey the host as dormant cells that hardly proliferate. They will get stimulated only in response to the specific threat against which they were raised. When called for duty, T cells enter a phase of massive growth and proliferation to generate a large amount of effector cells to fight off the threat. This dramatic change in T cells function is driven by a major reprogramming of their metabolism. We investigate how metabolism drives T cell activation, and how this transition is supported by the metabolic microenvironment in vivo.
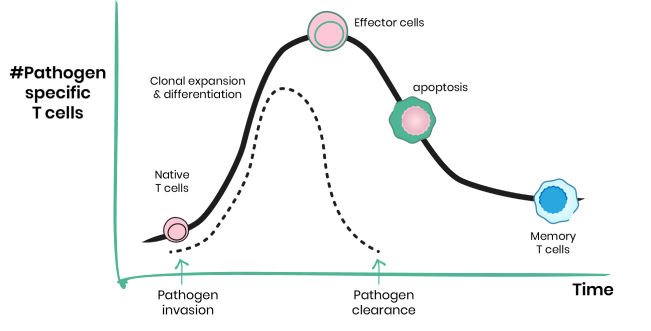
Discover metabolic drivers of T cell aging
Life expectancy has dramatically increased over the last century. Unfortunately, for many people, extended life span leads to longer suffering from age related diseases as aging negatively affects almost every system in our body. The immune system protects the host against intruders; however, it also plays a crucial, everyday role in identifying and responding to deviations from homeostasis or tissue damage. In fact, introducing dysfunctional T cells into young mice induces aging phenotypes in multiple organs. Our studies aim to discover how the aged microenvironment induces T cell dysfunction.
To accomplish our goals, we integrate multifaceted techniques and models spanning proteomics, metabolomics, cell and molecular biology, in vivo mouse immunology and human samples.